A Closer Look at Regrowth Patterns with Alopecia Areata
Baricitinib was approved for treating severe alopecia areata in June 2022. We have reviewed the pivotal BRAVE AA 1 and BRAVE AA2 studies that resulted in the drug’s FDA approval in the past.
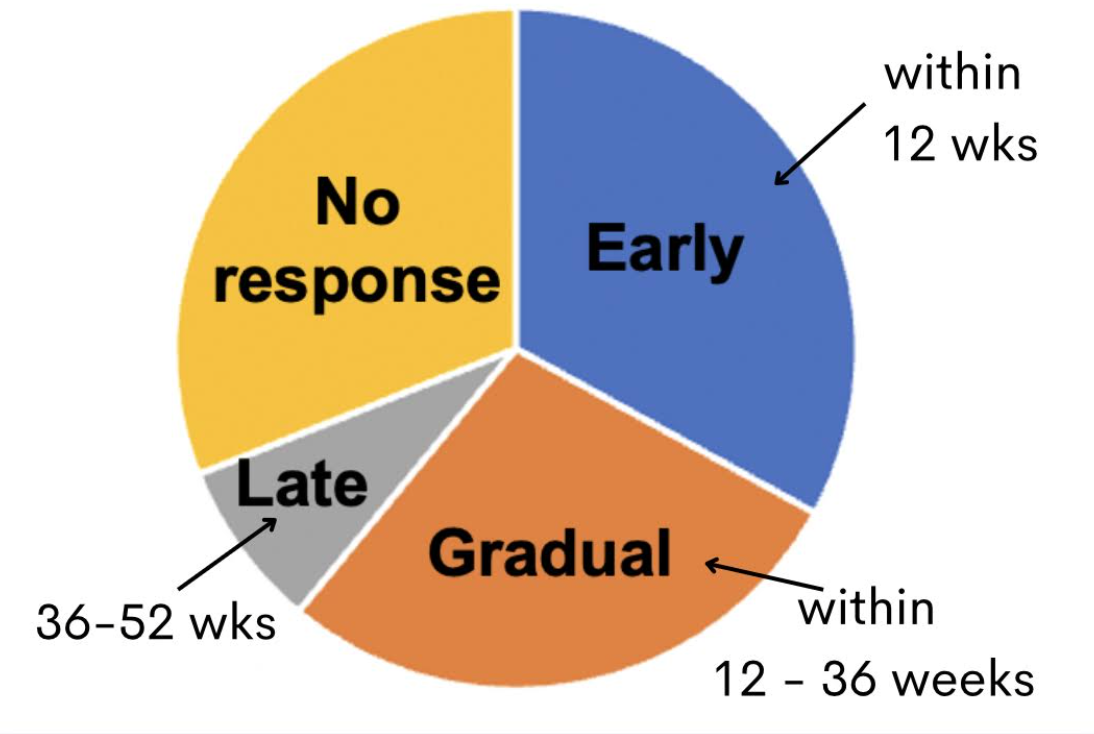
A new study examined when patients can expect to see the start of some significant regrowth. The authors used a SALT30 (30 % reduction in SALT score) as the key parameter to denote a trend towards good growth. 33 % of patients started experiencing good regrowth in the first 12 weeks. These were termed “early responders.” 28 % had the start of regrowth between week 12 and 36. These were “gradual responders.” 8 % had growth between week 36 and 52. These were termed “late responders.” 31 % of patients did not experience much in the way of regrowth at all with baricitinib and these were termed “non responders.” Early responders were more likely to be patients with less severe forms of alopecia areata. Patients with totalis and universalis were more likely to be late responders.
REFERENCE
King B et al. When to expect scalp hair regrowth during treatment of severe alopecia areata with baricitinib: insights from trajectories analyses of patients enrolled in two phase III trials. Br J Dermatol. 2023 Sep 14:ljad253.
This article was written by Dr. Jeff Donovan, a Canadian and US board certified dermatologist specializing exclusively in hair loss.