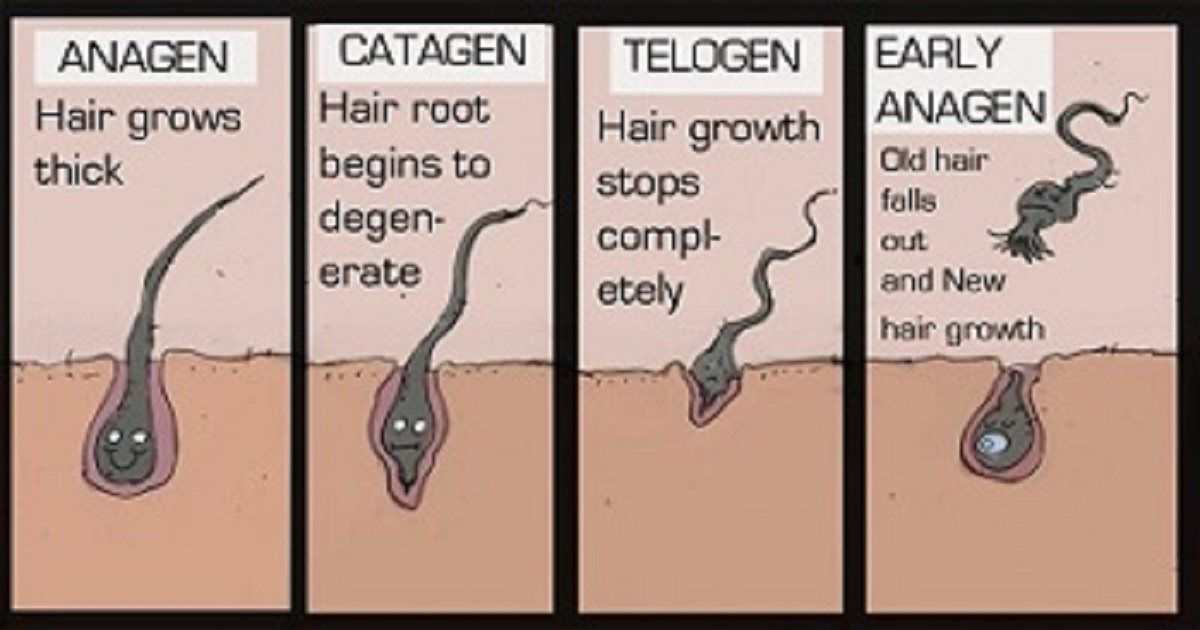
Veradermics VDPHL Tablet: Phase 2 Trials Begin
Veradermics is a US-based startup that is working on a new tablet to treat androgenetic alopecia (AGA). They just started Phase 2 clinical trials for VDPHL01 in male subjects with AGA. Only 20 patients are enrolled, and the completion date is listed as August 1, 2025. The trial will take place at Therapeutic Research’s center in San Diego, California. Also of interest, Therapeutic Research is conducting another hair loss product (topical) study at this very moment.
However, on their website, the pipeline page suggests that Phase 2 trials for VDPHL are finished (screenshot above). Thanks to reader “John Doe” for e-mailing me about this difference in time frame and acronym. In any case, this product is still a few years away from reaching the market assuming successful trial results. Note that VDPHL likely stands for Veradermics Pattern Hair Loss.
The tablet does not impact hormone levels as do dihydrotestosterone (DHT) inhibitors finasteride and dutasteride. Thus avoiding potential side effects. Veradermics’ CEO is a young dermatologist named Reid Waldman.
The mechanism of action (MOA) and key ingredient(s) in this tablet are both confidential. However, when I searched through the company’s patent, it seems like the drug candidate will be a “modified release” oral minoxidil tablet. In the patent, they use the term “extended release (ER)”.
Note that they do not use the term “sublingual minoxidil” anywhere in the patent. Also, I wonder how they will be able to differentiate from the existing oral minoxidil based “Hairy Pill” from Australian dermatologist Dr. Rodney Sinclair’s company?
Other Potential Ingredients
In the patent, they also have a massive list of 191 claims. Within that section, all of the following drugs are listed 11 times each:
- Setipiprant (11 times).
- Valproic acid (11 times).
- Cetirizine (11 times).
- Medrogestone (11 times).
For long time readers of this blog, setipiprant (and Kythera) will ring a bell. It caused so much excitement a decade ago. I cannot believe that the very optimistic 9-yr old audio interview with Kythera’s CEO is still online. Setipiprant is an oral antagonist to the prostaglandin D2 (PGD2) receptor.
I covered valproic acid and hair growth in detail in the past. Follica also has a patent that covers valproic acid and hair regrowth. Valproic acid activates the Wnt/?-catenin signaling pathway.
Cetirizine is a PGD2 inhibitor that has been shown to benefit hair growth even when used topically.
I have never covered medrogestone on this site before. Per Wikipedia, it is a progestin that is an agonist of the progesterone receptor and a weak anti-androgen. Progesterone is a female sex hormone that has beneficial properties towards hair growth.