Kintor Pharma: KX-826 and GT20029 for Androgenetic Alopecia
My original post on Kintor Pharmaceutical (China) has become way too lengthy after so many regular updates. So I will now add all new developments here. This rapidly moving well funded company is in Phase 2 and Phase 3 trials in the US and China for both its two androgenetic alopecia (AGA) products.
Kintor (pipeline here) is conducting hair loss trials for:
- Two separate androgen receptor (AR) targeting products: a degrader (GT20029) and an antagonist (KX-826 aka Pyrilutamide).
- Each of these trials is being conducted in both China and the US.
- Each of these products is being tested for both males and females with androgenetic alopecia (aka pattern hair loss).
i.e., a total of 8 types of clinical trials times 3 phases in each = 24 developments we have to track. A bit too much if all of these do end up taking place, but a most welcome development. Over one-half of these 24 potential trials are already finished.
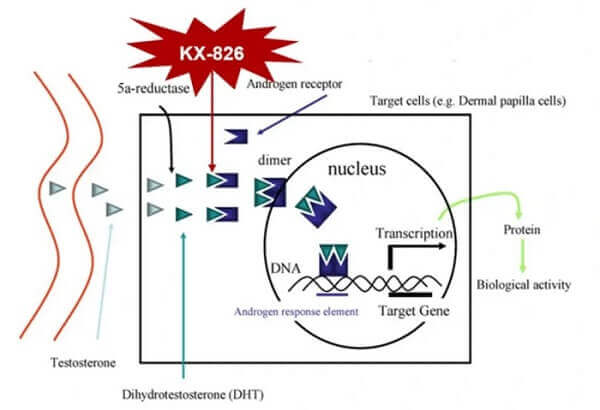
- The GT20029 product is an androgen receptor degrader (AR Degrader). It is developed using Kintor’s proprietary Proteolysis Targeting Chimera (PROTAC) platform. This is the world’s first topical androgen receptor (AR) compound (AR-PROTAC) to enter clinical trials. GT20029 degrades the AR protein via the E3 ubiquitin ligase pathway. During preclinical studies, GT20029 did not cause any notable side effects or systemic drug accumulation.
- Kintor’s main product for treating male pattern hair loss is KX-826 (Pyrilutamide) and is an androgen receptor antagonist (AR Antagonist). KX-826 is currently in Phase 3 clinical trials in both China and the US per the pipeline chart. In China, these trials are done (see my new update further below).
Note that Cassiopea’s Breezula (Clascoterone) is an AR antagonist that is also currently in Phase 3 trials in the US. Kintor’s website has an interesting article discussing both these competing AR antagonist products and hair loss.
Make sure to also read my related past post on destroying the androgen receptor to reverse hair loss.
Phase 3 Trials for KX-826 (Pyrilutamide)
KX-826 (Pyrilutamide) is currently in the most advanced stage and has a good chance of coming to market by the end of 2024. In Kintor’s pipeline page, it is shown to be in the final stage of Phase 3 trials for men in China; and early stage of Phase 3 trials for men in the US. Moreover, it is also in the early stage of Phase 3 trials for women in China.
Kintor is also undertaking a second Phase 3 trial in China for long-term safety of KX-826, which I discussed in detail in my original post. Even more exciting, one my Chinese readers sent me the below partial translation of a new October 2023 presentation by Kintor. He said that his English is not good, but I only changed a few words that were confusing or out of place.
”
KX-826 is a topical androgen receptor (AR) antagonist independently developed by Pioneer Pharmaceutical. It is the first AR antagonist in the world to enter phase III clinical trials for the treatment of hair loss. KX-826 binds the AR by competing with dihydrotestosterone (DHT) to locally block androgen-mediated signaling to limit hair follicle miniaturization and promote peripheral hair growth. After reaching the circulatory system, KX-826 is rapidly metabolized into inactivated metabolites, which has little influence on the whole body AR signaling pathway and has good safety.
The Phase II clinical trial in China included 120 men with hair loss. Of these, 90 subjects were randomly assigned to KX-826 0.25% twice daily, KX-826 0.5% once daily, and KX-826 0.5%, and the remaining 30 subjects were randomly assigned to placebo. After 24 weeks of treatment, the 0.5% concentration group of KX-826 showed a significant improvement in the amount of non-vellus hair in the target area. An increase of 22.73 roots per square centimeter compared to baseline.
Finally, news about Phase III: KX-826 is currently in or planned for five Phase III clinical trials (two in China and three in the United States). Among them, the Chinese Phase III clinical trial of KX-826 for male alopecia has completed the last subject visit, and the company is making full efforts to promote the data collation, library un-blinding and data statistical analysis of this clinical trial.
“
Phase 2 Trials for GT2009
In Kintor’s August 22, 2023 update, they announced the completion of patient enrollment in Phase II clinical trials of GT20029 for male pattern hair loss in China. The current pipeline shows that these trials are almost over in China, and close to beginning in the US.