Read the bottom half of this post for a history of Hope Medicine and Bayer’s HMI-115. This treatment targets the prolactin (PRL) receptor to cure hair loss. Hope Medicine (also called Heqirui Medicine) raised $56 million in funding in 2021. See their pipeline page for more information.
Read the bottom half of this post for a history of Hope Medicine and Bayer’s HMI-115. This treatment targets the prolactin (PRL) receptor to cure hair loss. Hope Medicine (also called Heqirui Medicine) raised $56 million in funding in 2021. See their pipeline page for more information.
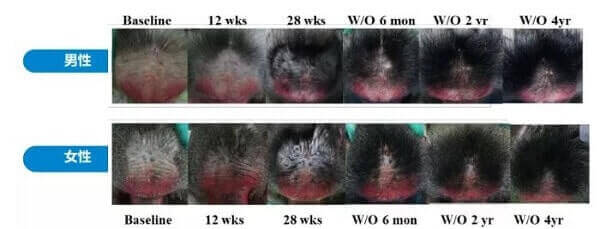
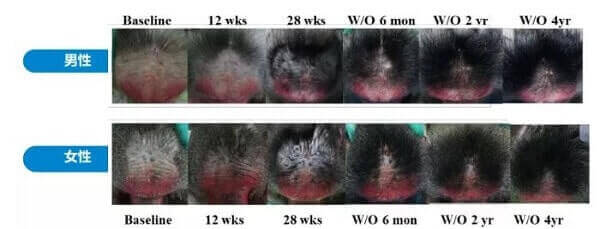
The initial reason for all this excitement was due to the spectacular long-lasting hair growth results seen in stump-tailed macaque monkeys. These monkeys are still being monitored and have not lost their hair even over four years after stopping prolactin receptor antibody treatment.
Update: October 27, 2023
Hope Medicine and Chime Biologics Enter Manufacturing Agreement
Great news! Hope Medicine (China) and Chime Biologics (China) have partnered to “speed up” the manufacture of the first-in-class monoclonal antibody drug HMI-115. Chime Biologics is a leading contract development and manufacturing organization (CDMO). The two companies are committed to commercialization of HMI-115 for the treatment of both androgenetic alopecia and endometriosis.
Update: August 1, 2023
Phase 3 Trials Begin in China
The HMI-115 Phase II clinical trials for androgenetic alopecia in China have now started.
Encouraging Updates from Australian Phase 1 Trial Participant
A November 2, 2022 detailed Reddit update from an Australian trial participant (HMI-115 dose 1 and dose 2) is required reading. This 37-year old male described in detail his first two doses of the prolactin blocker via injection. He (along with his 46-year old female partner) will get treatment every two weeks for six months. So a total of 12 doses, all at Sinclair Dermatology in Melbourne. Note that while these are Phase 1 trials in Australia, the same HMI-115 product is scheduled to undergo Phase 2 clinical trials in the US anytime soon.
This Reddit user “moeman32” seems legitimate and has a posting history of over ten years. He has also written about Australia many times in the past. He will do follow-up posts every two weeks. Note that all 20 patients in this Phase 1 safety trial are getting the actual drug injected. i.e., no placebo participants needed to test for efficacy this time around.
Update February 8, 2023: Unfortunately, he had to remove his posts due to some stalker issue. But the below threads have as yet not been deleted.
Update: April 12, 2022 — For some reason, Australian Phase 1 trials for Hope Medicine’s HMI-115 are starting today. They will involve 20 participants and are expected to be completed in July 2023. The trials will be led by the renowned hair loss expert Dr. Rodney Sinclair. And there is lots of excitement on Reddit.
Meanwhile, US Phase 2 trials should start at any moment now (read further below). This difference in trial progression by country is strange. Perhaps is has to do with some stricter Australian government requirements.
Update: January 26, 2022
Hope Medicine HMI-115 US Phase 2 Trials
Hope Medicine just received FDA approval to commence Phase 2 clinical trials in the US for its HMI-115 product to treat androgenetic alopecia. HMI-115 is a human monoclonal antibody drug that targets the PRL receptor for the treatment of male and female pattern hair loss. Last year, the US FDA also approved Phase 2 trials of the same drug to treat endometriosis.
The Phase II clinical trial of HMI-115 for the treatment of androgenetic alopecia:
“Will be an international multi-center, randomized, double-blind, placebo-controlled study. It will be carried out in the United States, Australia and other countries.”
Update: November 28, 2021
New CEO Henri Doods Interview
A reader sent me the below screenshot of Hope Medicine CEO Henri Doods’ Chinese magazine interview that I mentioned earlier. If anyone can translate into English, please post in the comments. Click on the image to expand.

Update: September 20, 2021

Reader “Karl” just notified us that there is a brand new cover page interview of Hope Medicine’s CEO Dr. Henri Doods in a Chinese magazine “tradetree.cn”. If anyone can gain access to it and translate into English, please try. Not worth purchasing as yet in my opinion.
Per Karl, Phase 2 clinical trials for hair loss product HMI-115 are planned for Q4 2021 (but no proof anywhere else). This matches what we expected per the prior updates that I discussed in May (see further below). Note that HMI-115 is the same as Bayer’s prolactin receptor antibody. Hope Medicine has the rights to the development of this product.
May 9, 2021
Hope Medicine (China) Raises $56 Million
Until now, Kintor Pharmaceutical (China) was the main reason for recent excitement. However, this weekend, the participants on this site’s hair loss chat are discussing Hope Medicine. Out of the blue, this startup company received $56 million in a Series B round of financing. Investment firms Qiming Venture Partners and Grand Flight Investment led the way.
Other investors include: HighLight Capital; Sinovation Ventures (a venture capital firm led by former Google China head Kai-Fu Lee); and Trustbridge. More here.
HMI-115 PRL Receptor Targeting: Bayer License
“Previously, HopeMed signed an exclusive license agreement with Bayer AG on the development and commercialization of a human antibody (HMI-115) targeting the prolactin (PRL) receptor for the treatment of male and female pattern hair loss. At present, HMI-115 has completed phase I clinical trials in the European Union, with positive safety results. The global multi-center phase II clinical trials for androgenic alopecia and will soon be launched.“

Note that I first covered Hope Medicine in my 2019 post on its partnership with Bayer in regards to the prolactin receptor. The company was founded by Dr. Rui-Ping Xiao, the dean of the College of Future Technology of Peking University. Apparently, she is also an associate editor of the New England Journal of Medicine. Update: See this page for much more on Dr. Xiao Ruiping.
The most unusual part of this story is that Hope Medicine’s official website (hopemedinc.com) has not worked for weeks.