A double dose of South Korea this week. First, a review of the popular Folligen hair loss shampoo from Dr.FORHAIR (South Korea). Then, an important positive new development in South Korea’s regenerative medicine sector.
Folligen Shampoo from Dr.FORHAIR
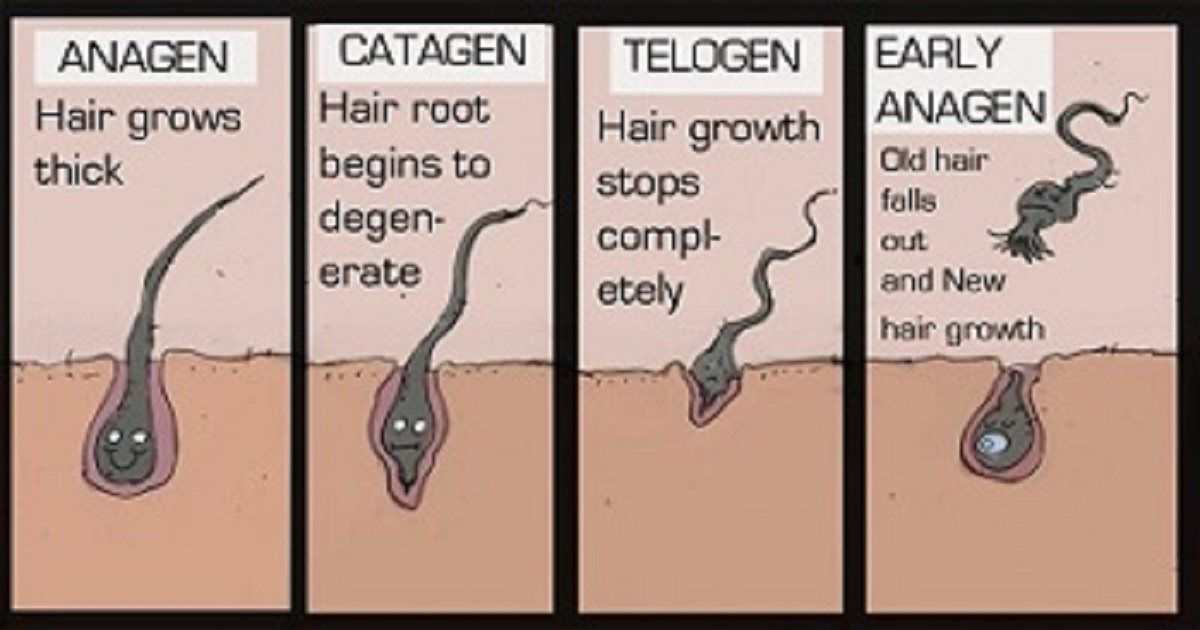
Folligen original biotin shampoo from Dr. Forhair (South Korea) is the most popular hair loss shampoo in South Korea. According to the product’s Amazon page, 13 million units of Folligen have been sold in just South Korea.
Among the key ingredients in this shampoo include biotin, saw palmetto extract and ceramides. This combination is called the Folligen Complex. Other ingredients include zinc pyrithione, panthenol and salicylic acid. All of these reduce dandruff and relieve scalp itching and flaking.
According to the manufacturer, Folligen can reduce scalp sebum by 78.4% and dead skin cells by 37.6% per clinical studies. It currently has an average rating of 4.4 out of 5 stars on Amazon, based on 425 reviews. Folligen shampoo was launched in Costco (US) in October 2023.
Folligen shampoo contains no parabens, sulfates or silicone. Note that Dr.FORHAIR also sells various other hair loss treatments and tonics on its website. This includes the Bio-3 shampoo.
Amendment to Regenerative Medicine Safety Act
In February 2024, South Korea passed a bill that adds regenerative medicine organizations to the list of those considered for human cell management business licenses. This will have positive implications when it comes to faster in-clinic use of new autologous hair loss treatments.
According to one article, this amendment to the Advanced Regenerative Biotech Act will serve as a positive catalyst for both South Korean and Japanese cell therapy companies. Note that Japan fast tracked its stem cell regenerative medicine rules a decade ago. Both South Korea and Japan suffer from rapidly aging societies with extremely low birth rates. Thus making regenerative medicine a topmost priority.
The title of the bill that South Korea’s National Assembly passed is:
“Amendment to the Act on Safety and Support for Advanced Regenerative Medicine and Advanced Biopharmaceuticals.”
This amendment will add regenerative medicine organizations to those considered for licensure to manage human cells. The cell management business entails the collection, importation, testing, and processing of human cells. These organizations supply cells as raw materials for advanced biopharmaceuticals.
In addition to existing manufacturers and umbilical cord blood banks, advanced regenerative medical institutions will now be recognized as licensees for managing human cells.
If the revised advanced regenerative biotech law is enforced (as expected):
“Advanced regenerative medical institutions with facilities, equipment, and manpower similar to the standards for licensing human cells and other management businesses will be recognized as licensed as a management business.”
Subsequently, advanced regenerative medical institutions will be allowed to supply raw materials to advanced biopharmaceuticals through simple separation, washing, freezing, and thawing of cells derived from patients.
Of special significance, this opens the way for actual patients (rather than just research subjects) to receive regenerative medicine services. Even if a South Korean patient is not a participant in a clinical trial, he or she can in the near future still receive cell therapy treatment.
I hope that these law changes will benefit the increasing number of new South Korean stem cell based hair loss treatment companies.