A few readers have recently mentioned that Follica’s homepage has gone dark. It now only displays a logo and contact e-mail. You can go through my Follica category section to see the numerous times that I covered this company in the past. Unfortunately, as was the case with Histogen, Replicel and Samumed, a decade of coverage has come to naught.

Note that Follica was founded all the way back in 2005. In 2019, they finally seemed close to beginning Phase 3 trials for their wounding (aka microneedling) device. With the addition of Minoxidil and other proprietary compounds into the wounded scalp skin. Even as recently as 2022, the company’s CEO Jason Bhardwaj sounded very optimistic.
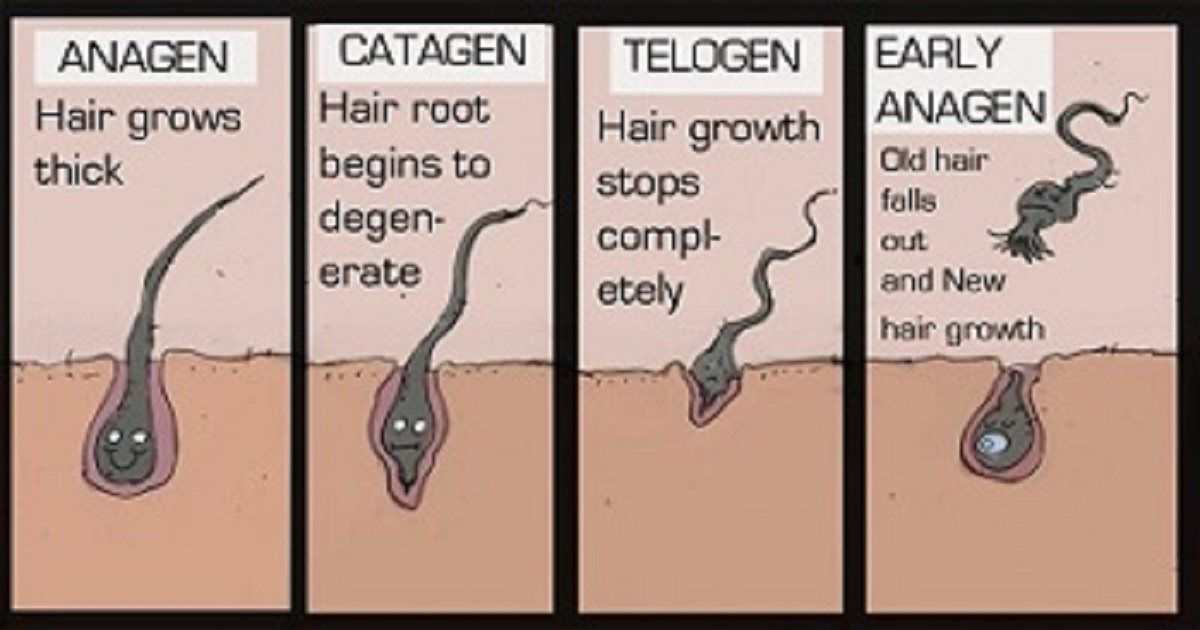
The following quote (credited to Dr. George Cotsarelis) from the old Follica website was very motivating. It meant an almost natural hair regrowth treatment that would likely face few regulatory hurdles from the US FDA:
“Following skin disruption, cells that migrate to help healing are forced to make a decision: Should I make epidermis, or should I make a hair? There is a window of opportunity in which we can potentially push them to choose the latter, and we believe there are multiple biological pathways to target to enhance this outcome. This regenerative effect is called hair follicle neogenesis.”
Follica’s technology is based on work originating from the University of Pennsylvania (led by Dr. Cotsarelis) that has been further developed by Follica’s internal research team.
Follica Goes Into Hibernation
I was not planning to write this post until a reader e-mailed me a recently uploaded 2022 Annual Report from PureTech Health (majority owner of Follica). In there are some interesting statements, the most relevant of which is as follows:
“With an increased focus on resource allocation towards our Wholly Owned Programs, we decided to hibernate the Follica Founded Entity in the 2023 post-period. We may choose to advance this program at a later date or with partners.”
- PureTech owns 85.4% of Follica.
Note that other companies such as OrganTech and Follicum have recently come back from the dead for second attempts. I feel like Follica will likely come back, although perhaps in a country with more favorable regulations than the US (see below)?
Cumbersome US Regulatory Process to Blame?
The above mentioned Annual Report breaks out in detail the numerous challenges that PureTech/Follica faced with the US government regulatory process. The two main issues seem to pertain to:
- Getting separate approvals for a drug versus a device. And from various distinct segments of the FDA.
- Complex rule differences governing “wholly-owned” versus “founded” entities. Follica is 85.4% owned by PureTech (after several increases in the stake over the years).
It does seem strange that PureTech is mentioning these issues so late in the game. Especially since they could have tried to bring their device to market with just topical Minoxidil (already FDA approved). I am assuming their proprietary drug contained more than just Minoxidil (a debate we had for many years on this site).
Below, I am pasting the entire statement from page 183 of their 2022 Annual Report:
“The complexity of a combination therapeutic that includes a drug or biologic and a medical device presents additional, unique development and regulatory challenges, which may adversely impact our or our Founded Entities’ development plans. And our or our Founded Entities’ ability to obtain regulatory clearance, authorization or approval of our Wholly Owned Programs or our Founded Entities’ therapeutic candidates.
We or our Founded Entities, such as Follica, may decide to pursue marketing authorization of a combination therapeutic. A combination therapeutic may include, amongst other possibilities, any investigational drug, device, or biologic packaged separately that according to its proposed labeling is for use only with another individually specified investigational drug, device, or biologic where both are required to achieve the intended use, indication, or effect.
Developing and obtaining regulatory clearance, authorization or approval for combination therapeutics pose unique challenges because they involve components that are regulated by the FDA under different types of regulatory requirements, and by different FDA centers. As a result, such therapeutics raise regulatory, policy and review management challenges.
For example, because divisions from both FDA’s Center for Drug Evaluation and Research or Center for Biologics Evaluation and Research and FDA’s Center for Devices and Radiological Health must review submissions concerning therapeutic candidates that are combination therapeutics comprised of drug or biologics and devices, respectively, the regulatory review and clearance, authorization or approval process for these therapeutics may be lengthened.
In addition, differences in regulatory pathways for each component of a combination therapeutic can impact the regulatory processes for all aspects of therapeutic development and management, including clinical investigation, marketing applications, manufacturing and quality control, adverse event reporting, promotion and advertising, user fees and post-clearance, authorization or approval modifications.
Similarly, if applicable, the device components of a combination therapeutic candidate will require any necessary clearances, certifications or approvals or other marketing authorizations in other jurisdictions, which may prove challenging to obtain.”