I have covered Japanese cosmetics behemoth Shiseido for almost ten years across over a dozen posts. My last post about them became too confusing, as I was regularly appending it with brief new updates. I was awaiting the correct time to restart.
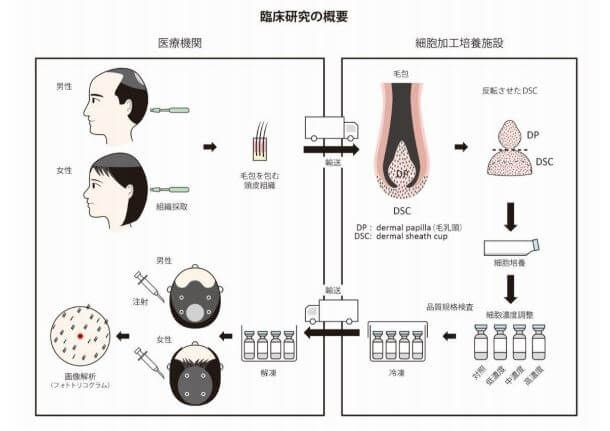
Shiseido’s work on a hair loss cure based on the injection of cultured autologous dermal sheath cup cells (DSCs) is promising and safe. While the company has no problems with fundraising, thay have had some issues with their partnership with Replicel (Canada). I will not repeat that story here, but readers can check out my past posts on those two companies for more details.
Shiseido Phase III Study Results Published
Earlier this month, Shiseido released the results of its “Phase III equivalent” study. The actual work was published in “The Journal Of Dermatology” Volume 50, Issue 10, in December 2023. In that same second link above, it also says “First published: 26 September 2023”, which might mean date of original Japanese submission.
No-one seems to have noticed it on Reddit as far as I can tell. I only learnt about it due to someone e-mailing me and asking me to check the end of the first PubMed link above where it says: “Grants and funding: Shiseido Group.”
It is not surprising that this development went through without notice. For one, the title of the paper does not even mention Shiseido’s name:
“Efficacy of autologous dermal sheath cup cell transplantation in male and female pattern hair loss”.
Even causing more confusion is the fact that they call this a “Phase III equivalent clinical study.” And only 36 volunteers participated in this trial. Not even remotely close to what you expect in a proper large-sized Phase 3 trial. The Shiseido website’s Hair Regenerative Medicine page has no new updates either.
In any event, this is clearly Shiseido’s trial for its DSC injections. After culturing dermal sheath cup cells (the source of dermal papilla cells) from the scalp skin of the hair loss patient, they are implanted (injected) into the same patient’s balding scalp skin to rejuvenate damaged hair follicles and promote healthy hair growth.
Among the paper’s many co-authors are the renowned Shiseido-affiliated Dr. Ryoji Tsuboi and Dr. Manabu Ohyama. All of the co-authors are also associated with at least one of the folllowing four entities in Japan:
- Department of Dermatology, Tokyo Medical University, Tokyo.
- Department of Dermatology, Kyorin University Faculty of Medicine, Tokyo.
- Department of Dermatology, Toho University Ohashi Medical Center, Tokyo,.
- Regenerative Medicine Research & Business. Development Department, Yokohama.
Results
Keeping in mind the small size of the study, the results while positive, are a bit underwhelming. Thirty-six male and female participants with pattern hair loss (PHL) were injected with dermal sheath cup cells. These DSCs were harvested from non-affected occipital hair follicles twice in quarterly intervals.
On global photographic assessment, 30% of the participants showed improvement.
The phototricogram data analysis showed increases in the:
- Cumulative hair diameter of 107.6 ± 152.6 ?m/cm2. This was a +1.4% increase versus baseline.
- Hair cross-sectional area of 3069.1 ± 10960.7 ?m2 /cm2. This was a +3.4% increase versus baseline.
- Mean hair diameter of 0.9 ± 0.9 ?m. This was a +2.2% increase versus baseline.
The female and high terminal hair ratio groups achieved better outcomes. It is not clear if any new hair growth occurred from the above breakouts. But stronger and denser existing hair after 12 months is a positive. And could imply better future protection against dihydrotestosterone (DHT) spurred follicle miniaturization.
Another confusing statement:
Of the total participants, 62.9% noted some degree of improvement.
It seems like this 62.9% figure is based on patient feedback rather than any kind of measurement. And per the math, this would imply that 22 of 35 patients gave a positive response, while 1 dropped out before the 1-year mark.
I have not tried to find any long version of this report, as overall it leaves much to be desired. I hope a far lengthier Phase 3 clinical trial is still going on.
And I also hope that Shiseido will be able to remain fully transparent, in spite of any pending legal issues with Replicel.