Researchers have announced a new hair loss study in relation to a potential treatment for the autoimmune condition, Alopecia Areata.
A clinical trial due to start towards the end of November 2015 will explore whether Secukinumab (brand name: Cosentyx) – a drug which is currently FDA-approved for the treatment of another autoimmune condition, rheumatoid arthritis – could prove effective in treating Alopecia Areata in men and women.
The clinical trial titled ‘An Exploratory Study to Evaluate the Safety and Efficacy of Secukinumab in the Treatment of Extensive Alopecia Areata’ was registered this month but is not yet recruiting. It follows the promising results the rheumatoid arthritis JAK inhibitor, Xeljanz (tofacitinib) is showing in on-going trials for the treatment of severe Alopecia Areata.
Research participants
 This new, interventional study is due to be carried in America at New York’s Icahn School of Medicine at Mount Sinai, with the research being funded by Novartis, the pharmaceutical company which produces Cosentyx.
This new, interventional study is due to be carried in America at New York’s Icahn School of Medicine at Mount Sinai, with the research being funded by Novartis, the pharmaceutical company which produces Cosentyx.
Now in its second phase, it will involve 30 participants who have each had at least 60 per cent of their scalp affected by Alopecia Areata for a minimum of 6 consecutive months prior to the study start date. There will be no healthy controls used as part of the trial.
Participants will be a mix of men and women, all of whom will be over 18 years of age and fit a number of qualifying criteria. These include being in generally good health, having no additional hair loss conditions, and women must not be pregnant or nursing,
Additional disqualifying factors include having active Crohn’s disease, a known hypersensitivity to latex, being HIV, HCV or HBV positive, having previously used Secukinumab or any other drug targeting IL-17A or its receptor; or having used topical or immunosuppressant treatment for Alopecia Areata within the month prior to the trial.
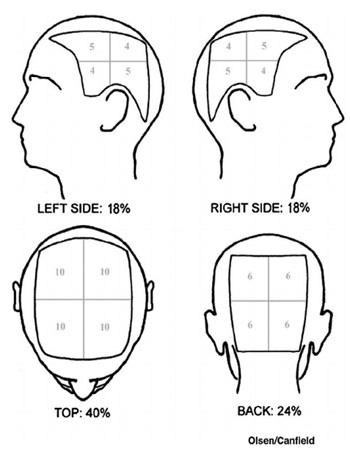
An Example of How Alopecia Areata SALT Scores are Allocated
Double-blind study
Each of the trial participants will receive ‘treatment’ injections at weeks 0, 1, 2, 3, 4 and every 4 weeks thereafter up to and including Week 20. For half the group – allocated on a blind and randomised basis – these injections will deploy subcutaneous secukinumab in 300 mg doses, whilst for the other half a placebo substance will be administered in the same amounts.
Three measures will be employed to assess the efficacy of the treatment on each individual. The first of these involves allocating a ‘Severity of Alopecia Tool’ (SALT) score by taking a reading from this widely-used scale to determine the level of each participant’s hair loss based on how much of their scalp is affected. The SALT calculation method was co-devised by Elise A. Olsen of the Department of Dermatology and Medicine (Oncology) at Duke University in North Carolina, USA, and is also sometimes referred to as the Olsen/Canfield tool.
Secondly each participant will be subject to examination and awarded a Physician’s Global Assessment (PGA) score, followed by a Dermatology Life Quality Index (DLQI) score. Both the PGA and DLQI scores will be allocated using a range from 0 (no regrowth) to 5 (100 per cent regrowth).
All these results will be measured at the beginning of the trial, then at weeks 24 and 28, following the subjects’ 20-week course of injections.
Timeline for results
The trial will take place over a 28-week period with results being analysed after this time; the estimated completion date for collecting all data from this phase of the trial is currently set at November 2016.
 Once this information has been collated and processed, it is likely the trial would go into the next phase of larger, wider-scale trials so – as with developing new hair loss treatments or medication of any kind – this may take years yet to determine the true safety and efficacy of Secukinumab in relation to treating Alopecia Areata.
Once this information has been collated and processed, it is likely the trial would go into the next phase of larger, wider-scale trials so – as with developing new hair loss treatments or medication of any kind – this may take years yet to determine the true safety and efficacy of Secukinumab in relation to treating Alopecia Areata.
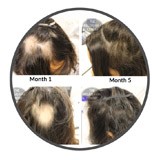
For those experiencing the patchy hair loss synonymous with Alopecia Areata now, there is help available in the meantime. Many Belgravia clients have responded well to topical treatment using high strength minoxidil applied directly to the areas where their hair has fallen out. Examples of a number of these clients can be seen pictured before and after starting their treatment plans in our Alopecia Areata Treatment Success Stories gallery.
 The Belgravia Centre—————————————————————————————————–
The Belgravia Centre—————————————————————————————————–
The Belgravia Centre is the leader in hair loss treatment in the UK, with two clinics based in Central London. If you are worried about hair loss you can arrange a free consultation with a hair loss expert or complete our Online Consultation Form from anywhere in the UK or the rest of the world. View our Hair Loss Success Stories, which are the largest collection of such success stories in the world and demonstrate the levels of success that so many of Belgravia’s patients achieve. You can also phone 020 7730 6666 any time for our hair loss helpline or to arrange a free consultation.